
Compass Pathways, the UK-based mental health care company that’s innovating new mental health treatments, recently announced that it had been granted two new patents in the US. These patents cover the use of its oral formulations of synthetic psilocybin, the active ingredient in magic mushrooms, to treat major depressive disorder (MDD) and treatment-resistant depression (TRD). The new patents join Compass’ first patent, granted for its methods of using COMP360, a synthetic psilocybin formulation, for TRD.
var axel = Math.random() + “”; var a = axel * 10000000000000; document.write(‘
‘);
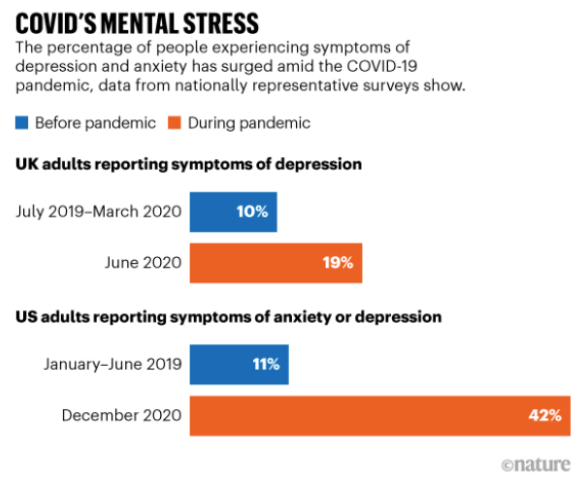
The possibility of finding a safe and effective treatment for depression is a major factor motivating both researchers and investors in the psychedelic market. It’s estimated that over 300 million people around the world, including more than 17 million Americans, have experienced serious depression at some point in their lives, and that 30%, or close to one in three patients, don’t respond to any of the current treatment options. The percentage of the adult population with serious depression rose from 6.1% in 1996 to 10.4% in 2015, but COVID-19 has caused those rates to skyrocket. Loneliness, bereavement, anxiety and fear over the risks of infection drive depression, as does the uptick in domestic violence and abuse which occurred during the pandemic. It’s also thought that long covid increases depression disorders. A study by the US Census Bureau found that the number of Americans reporting anxiety or depression jumped from 11% in December 2019 to 42% in December 2020, and from 10% to 19% in the UK.
The need for new approaches to treating mental health disorders is widely recognized, because the current ones simply aren’t working. Disillusion in the current treatment options is clear from the actions of the industry’s major players. The patents for existing medications for depression and anxiety will be expiring soon, and far from stepping up their research, big pharma companies are reducing their budget for mental health R&D. Patients also lack confidence that the current options could be effective, so it seems that the time has come for bold thinking. Compass is leading research into the use of psychedelics for depression. George Goldsmith, Compass’ CEO and co-founder, observes that there “is really a significant need for new models of care in mental health, where the current model isn’t serving enough people. I think that there is … a real need for innovation, and unmet need and innovation go hand in hand.”
As the need for new treatments has grown, psychedelics are shedding their reputation as a dangerous drug used by people looking to get high. A Data Bridge market research report states that “growing acceptance of psychedelic drugs for treating depression across the U.S. is one of the prominent factors for an upsurge demand of [sic] psychedelic drugs.” A number of cities and states in the US are decriminalizing psilocybin, with Oregon legalizing it entirely for therapeutic use and a similar bill making it through its second Senate hearing in California. The medicinal use of psychedelics is becoming part of the conversation, with Marc Aspinall writing in Wired that “psychedelic medicine will begin to cross over into the mental health mainstream in 2021.” There’s now a Center of Psychedelic Research at Imperial College, London, with a former chief drug advisor to the UK Government serving as its deputy head; Johns Hopkins University established a center for “psychedelics and consciousness research”; and a “center for psychedelic science and public education” was established at UC Berkeley in California in 2020. The leading non-profit research center Multidisciplinary Association for Psychedelic Studies (MAPS) raised $30 million last year to fund a phase-3 clinical trial for treating PTSD with MDMA.
All these developments are driving interest in investment in the psychedelics sector and fueling the performance of psychedelics stocks. While Compass Pathways was the first psychedelics stock to list on the NASDAQ, with a valuation of $1.6 billion, it’s now been joined by MindMed in April 2021, and Atai is talking about following suit, demonstrating the strength of the market. Compass has seen its US-listed shares rise around 165% since its IPO in September 2020, and in September 2020, the company announced that it had $196.5 million of funds, enough to carry out its operations until 2023. On top of that, Compass ran a second round of funding in April 2021, raising approximately $80 million from investors who include PayPal founder Peter Thiel. It announced that the latest funding will be used for exploring new uses for psilocybin therapy, advancing preclinical candidates, developing digital technologies that could be used alongside COMP360, and establishing new research partnerships.
Compass Pathways was formed in 2016 as a mental healthcare company aiming to develop new therapies for mental health issues. The co-founders experienced first-hand the difficulties of finding effective healthcare for serious depression. Compass’ first area of research is psilocybin therapy, and it’s already traveled a long way down the road to success, with three patents for various forms of psilocybin therapy.
The patents cover the method of administering psilocybin at least as much as the drug itself, as Compass is highly aware of the importance of the setting for psychedelic treatments. The company has ringfenced some of its funds for a treatment center within the Sheppard Pratt psychiatric hospital, converting clinical rooms into calming, therapeutic spaces to avoid triggering a “bad trip,” which can occur if someone has a frightening experience during psychedelic treatment. The Compass approach includes preparation sessions with a therapist before medication, followed by administration of psychedelic drugs in a soothing setting, with the patients lying on a couch wearing eye masks and listening to an ambient music playlist. It’s designed to help them focus on the experience and heighten the impact of the medication. After the treatment itself, patients are invited to talk with a therapist. Wilde says “We know that these experiences can go wrong when they aren’t done in a therapeutic setting. “So it’s important that we figure out how to deliver this therapy safely, in a scalable form.”
Compass completed phase one clinical trials for COMP360 in December 2020 through King’s College London. The trial results showed the substance is safe, feasible, and well tolerated by healthy users, with no participants showing any negative effects on their cognitive or emotional functioning. Phase 2b trials are underway, with results initially expected in 2021, but it’s likely that they’ll be delayed due to the impact of the coronavirus pandemic. In 2018, Compass won breakthrough status from the FDA for it’s COMP360 psilocybin treatment for TRD.
Alongside clinical trials, Compass is driving research and development for new mental health treatments and methodologies. In February 2021, the company announced the expansion of its Drug Discovery Center for research into psychedelic substances, to include collaborations with laboratories at UC San Diego, School of Medicine, and Medical College of Wisconsin (MCW). The Discovery Center was first established in August 2020, when Compass paired with Jason Wallach, assistant professor of pharmaceutical sciences at the University of the Sciences in Philadelphia, to investigate new serotonergic compounds such as the active ingredients in psilocybin, DMT, mescaline, ibogaine and LSD.
The two new patents cover Compass’ claims for pharmaceutical formulations and for oral dosage of crystalline psilocybin for treating MDD. The company’s very first patent was granted in December 2019 for Compass’ COMP360, to be used in specific therapeutic applications for people with TRD. It’s important to note that the patent isn’t for psilocybin itself, because that is a known natural compound and cannot be patented, but for Compass’ method of making and utilizing the substance. The patent gives Compass intellectual property protection for COMP360 for up to 7.5 years in the US and up to 11 years in Europe. The patents are controversial, and opposed by rival psychedelic companies who are nervous about a monopoly over psilocybin research. Lars Christian Wilde, chief business officer of Compass Pathways, insists that they wouldn’t act to squash any research from a different organization, but the first Compass patent was still challenged repeatedly by the non-profit Usona Institute, causing it to take 14 months for approval to come through.
Wilde points out that patents play an important role in helping the company attract funding, which is vital for advancing psilocybin research and development. The sector is still new, and many investors are nervous about the risk involved in buying psychedelic stocks. A patent helps reassure investors about the chances that they’ll see returns on their investment. Writing in GlobeNewsWire, analyst David Kugelman says that “Compass Pathways’ biggest draw is its patent.” Another option for mitigating exposure to risk within this growing sector is to invest in a psychedelics ETF like Defiance’s PSY. By spreading investment across a number of promising new psychedelics stocks, interested inventors can take part in the new and potentially disruptive moves within the larger pharma market, while avoiding putting all their eggs into one young basket.
Investing involves risk. Principal loss is possible. As an ETF, the fund may trade at a premium or discount to NAV. Shares of any ETF are bought and sold at market price (not NAV) and are not individually redeemed from the Fund. The Fund is not actively managed and would not sell a security due to current or projected under performance unless that security is removed from the Index or is required upon a reconstitution of the Index.
A portfolio concentrated in a single industry or country, may be subject to a higher degree of risk. Specifically, the Index (and as a result, the Fund) is expected to be concentrated in Psychedelics Healthcare and Medical Cannabis companies. Such companies may depend largely on the government regulation and their profitability can be significantly affected by restriction on government reimbursements for medical expenses, rising costs of products and services, pricing pressure, limited product lines, intellectual property rights, and long and costly government product approval processes. The investments rely on U.S. and Canadian regulation of psychedelic, healthcare and cannabis, and the fund could be adversely affected by changes in these regulations.
The Fund is considered to be non-diversified, so it may invest more of its assets in the securities of a single issuer or a smaller number of issuers. Investments in foreign securities involve certain risks including risk of loss due to foreign currency fluctuations or to political or economic instability. Small and mid-cap companies are subject to greater and more unpredictable price changes than securities of large-cap companies.
The BITA Medical Psychedelics, Cannabis, and Ketamine Index is a rules-based index that tracks the performance of companies, listed in North American Exchanges, that operate business models focused on the usage of Psychedelics, Medical Cannabis, and Ketamine for medicinal and health treatment purposes. The index is owned, calculated, administered and disseminated by BITA GmbH.
PSY is new with a limited operating history.
Go to defianceetfs.com/PSY to read more about PSY including current performance and holdings information.
The Defiance ETFs are distributed by Foreside Fund Services, LLC.
The Funds’ investment objectives, risks, charges, and expenses must be considered carefully before investing. The prospectus contains this and other important information about the investment company. Please read it carefully before investing. A hard copy of the prospectus can be requested by calling 833.333.9383 or at defianceetfs.com.
See more from Benzinga
© 2021 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.