Overall, 22 138 citations and 3542 full text articles were screened (fig 2) and 256 articles (28 483 people with dementia) included in the systematic review (see supplementary references). Of 29 study authors emailed, 12 responded (41%): two provided additional data and one clarified study methods.343536 The Cornell scale for depression in dementia was the most reported outcome measure (table 1 and supplementary table 1). Most studies enrolled at least 50% of women and the mean age in most studies was at least 70 years. Studies were most often conducted in community and clinic settings (table 1 and supplementary tables 3a, 3b, 4a, and 4b).
People with dementia without a major depressive disorder
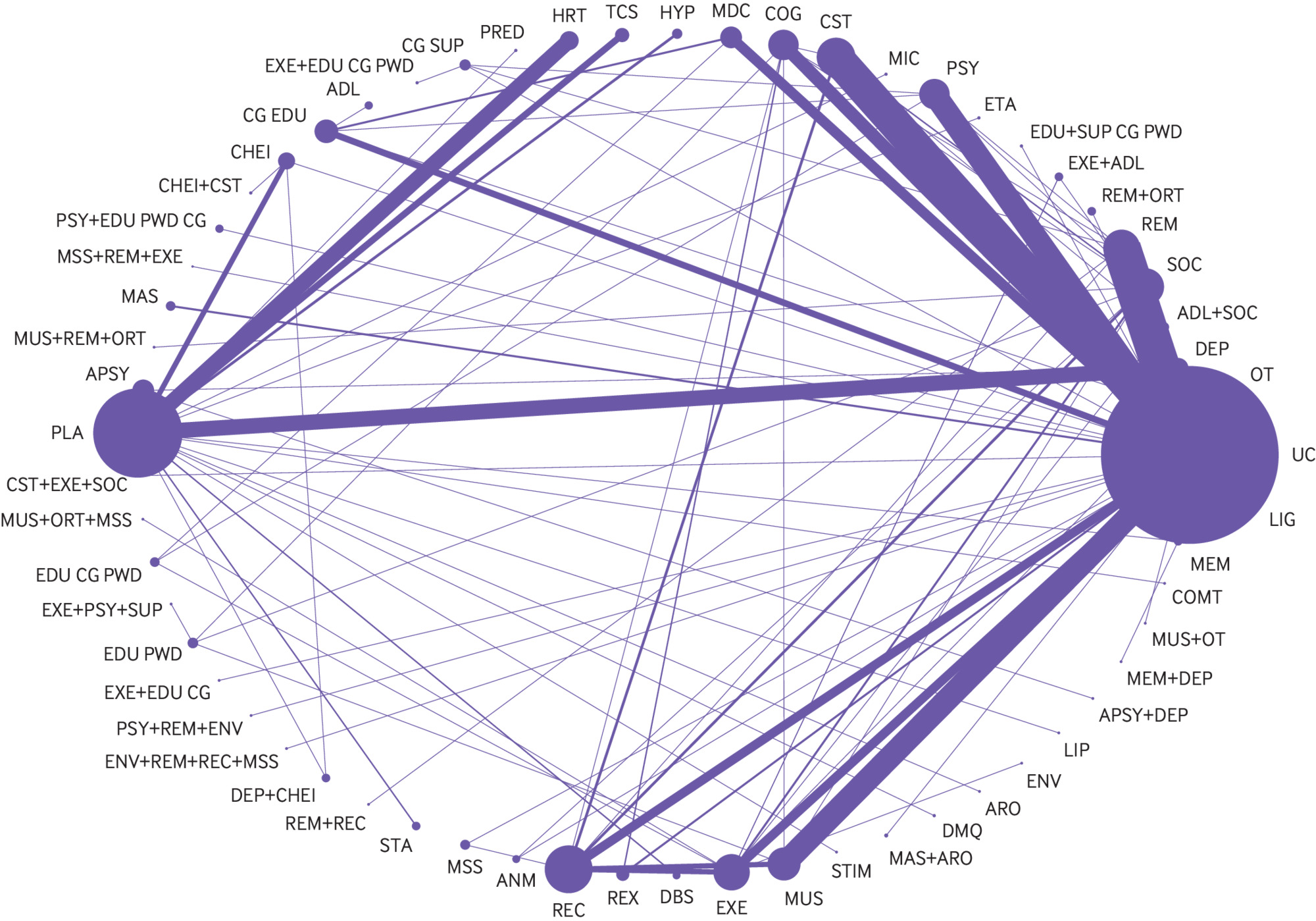
Two hundred and thirty five studies reported outcomes for interventions that targeted symptoms of depression in people with dementia without a diagnosis of a major depressive disorder. Of these studies, 213 (25 177 people with dementia) were included in pairwise meta-analysis and NMA. The network contained 61 connected intervention nodes: 70% of direct treatment comparisons involved usual care or placebo (fig 3). There were 45 triangular loops and seven quadratic loops. In most treatment comparisons, studies assessed symptoms of depression using the Cornell scale for depression in dementia and enrolled more women than men. Non-drug interventions were studied in 70% of trials. Nine studies compared the efficacy of an antidepressant with placebo and two studies compared the efficacy of an antidepressant with an antipsychotic.12132537383940414243 Risk of bias from missing data and blinding of outcome assessors posed the greatest risk to review findings: 42% of randomised controlled trials were at high risk of bias from missing data and 37% of trials were at unclear or high risk of bias from lack of assessor blinding (see supplementary table 5a and figure 1a). Differences were found in effect modifiers across 94 direct treatment comparisons—namely in terms of study setting, intervention duration, type of dementia, dementia severity, and risk of bias from missing data and lack of assessor blinding (see supplementary table 6). Standard deviation values associated with baseline mean Cornell scale for depression in dementia scores were included from 70 randomised controlled trials in the derivation of minimum clinically important differences. The estimated minimum clinically important difference for the Cornell scale for depression in dementia was 2.0 at 0.4 standard deviations and 2.5 at 0.5 standard deviations.
Network diagram for a network of interventions targeted at reducing symptoms of depression in people with dementia without a major depressive disorder. Nodes represent individual interventions, and nodes connected by lines indicate that the two connected interventions were directly compared in a study. Nodes are weighted by the number of studies that evaluated the treatment, and lines are weighted by the number of studies that evaluated the treatment comparison. ADL=activities of daily living modification; ANM=animal therapy; APSY=antipsychotics, ARO=aromatherapy, CG SUP=care giver support; CHEI=cholinesterase inhibitor; COG=cognitive rehabilitation; COMT=catechol-O-methyltransferase inhibitor; CST=cognitive stimulation; DBS=deep brain stimulation; DEP=antidepressant; DMQ=dextromethorphan-quinidine; EDU+SUP CG PWD=education and support of person with dementia and care giver; EDU CG=care giver education; EDU CG PWD=education of care giver and person with dementia; EDU PWD=education of person with dementia; ENV=environmental modification; ETA=etanercept; EXE=exercise; HR=hormonal therapy; HYP=antihypertensive; LIG=light therapy; LIP=lipid lowering therapy; MAS=massage therapy; MDC=multidisciplinary care; MEM=memantine; MIC=antimicrobial; MSS=multisensory stimulation; MUS=music therapy; ORT=reality orientation; OT=occupational therapy; PLA=placebo; PRED=prednisone; PSY=psychotherapy; REC=recreation therapy; REX=relaxation therapy; REM=reminiscence therapy; SOC=social interaction; STA=mood stabiliser; STIM=stimulant; TCS=transcutaneous stimulation; UC=usual care
Supplementary table 7 reports the pairwise meta-analysis and NMA outcomes. The between study variance (τ2) in the primary NMA was moderate (0.23, 95% credible interval 0.17 to 0.31). A consistency rather than an inconsistency model provided a better model fit (see supplementary table 7). Two closed network loops were inconsistent in the primary NMA (2/52 loops, 4%): usual care-social interaction-animal therapy (inconsistency factor 1.88, 95% confidence interval 0.93 to 2.84) and music therapy-social interaction-cognitive rehabilitation (1.33, 0.08 to 2.58) (see supplementary figure 2a). No evidence was found of small study effects (see supplementary figure 3).
In the primary NMA, cognitive stimulation, cognitive stimulation combined with a cholinesterase inhibitor, exercise combined with social interaction and cognitive stimulation, massage and touch therapy, multidisciplinary care, occupational therapy, and reminiscence therapy were found to be more efficacious than usual care for reducing symptoms of depression in people with dementia without a diagnosis of a major depressive disorder (table 2 and supplementary table 7). In pairwise meta-analysis, animal therapy, exercise, and psychotherapy combined with reminiscence therapy and environmental modification were also found to be more efficacious than usual care for reducing symptoms of depression in people with dementia (table 2 and supplementary table 7). Except for massage and touch therapy, cognitive stimulation combined with a cholinesterase inhibitor, and cognitive stimulation combined with exercise and social interaction, which were found to be more efficacious than some drug interventions, there was no statistically significant difference in the comparative efficacy of drug and non-drug interventions for reducing symptoms of depression in people with dementia without a diagnosis of a major depressive disorder (see supplementary table 7). These findings were unchanged when a minimally informative prior was implemented for the heterogeneity variable. Based on surface under the cumulative ranking curve values, the most highly ranked interventions were cognitive stimulation combined with exercise and social interaction (98.3%, 88.3% to 100%), cognitive stimulation combined with a cholinesterase inhibitor (98.3%, 95% credible interval 86.7% to 100%), and massage and touch therapy (95.0%, 86.7% to 100%). When a minimally informative prior was implemented for the heterogeneity variable, the most highly ranked interventions were cognitive stimulation combined with a cholinesterase inhibitor (98.3%, 88.3% to 100%), cognitive stimulation combined with exercise and social interaction (98.3%, 86.7% to 100%), and massage and touch therapy (95.0%, 85.0% to 100%).
Efficacy of interventions for reducing symptoms of depression in people with dementia without a diagnosis of major depressive disorder
In the NMA of studies with dementia diagnosed using standard criteria (eg, DSM-V), a consistency rather than inconsistency model provided a better model fit, and no inconsistent loops of evidence were found (see supplementary figure 2d). As in the primary NMA, cognitive stimulation, cognitive stimulation combined with a cholinesterase inhibitor, cognitive stimulation combined with exercise and social interaction, massage and touch therapy, multidisciplinary care, occupational therapy, and reminiscence therapy were found to be more efficacious than usual care (table 3 and supplementary tables 8 and 9). In this NMA of studies where dementia was diagnosed using standard criteria, massage and touch therapy, cognitive stimulation combined with a cholinesterase inhibitor, and cognitive stimulation combined with exercise and social interaction were found to be more efficacious than some drug interventions (see supplementary table 8). An antidepressant combined with a cholinesterase inhibitor was found to be more efficacious than care giver support (mean difference −9.2, 95% credible interval −18.3 to −0.08) in this subgroup of studies, but no statistically significant differences were found when the efficacy of other drug and non-drug interventions were compared (see supplementary table 8).
Efficacy of interventions for reducing symptoms of depression in subgroups of people with dementia without a diagnosis of major depressive disorder
People with dementia and a major depressive disorder
Twenty two studies (1829 people with dementia) reported outcomes for interventions aimed at reducing symptoms of depression in people with dementia and a major depressive disorder. The greatest risk to review findings was from missing data: 73% of studies were at high risk of bias from missing data (see supplementary table 5b and figure 1b). Too few studies and anticipated substantial heterogeneity among study characteristics and interventions precluded pairwise meta-analysis or NMA (see supplementary tables 3b and 4b). Studies were conducted in both community and non-community settings and enrolled participants with Alzheimer’s disease or unspecified types of dementia. Drug interventions included mirtazapine, sertraline, venlafaxine, fluoxetine, citalopram, escitalopram, desipramine, imipramine, clomipramine, amitriptyline, and paroxetine.164299100101102103104105106107108109 Seven randomised controlled trials reported the comparative efficacy of selective serotonin reuptake inhibitors (ie, sertraline, fluoxetine, citalopram, escitalopram) and placebo, with mixed conclusions.1699102103105106108 Neither mirtazapine nor venlafaxine were associated with improved symptoms of depression compared with placebo.16100 Multidisciplinary care was found to be more efficacious than usual care in one randomised controlled trial, but evidence supporting the efficacy of other non-drug interventions—namely, psychotherapy and exercise—was mixed.110111112113114115116117 Drug strategies for pain management (ie, paracetamol (acetaminophen) or buprenorphine) were not found to be superior to placebo.118